Optimized batteries via Spatial Atomic Layer Deposition
Spatial Atomic Layer Deposition is a thin film deposition technique that involves the sequential exposure of a substrate to different gaseous reactants called precursors resulting in coatings with atomic-scale precision.
Spatial ALD can be utilized in various applications, including to improve the performance and cycle-life of batteries.
Batteries: a closer look
Batteries are electrochemical devices that store and release energy. They have four main parts: the anode, the cathode, an electrolyte, and a separator. These components work together to store and release electrical energy that powers devices, often used in portable electronics and electrical vehicles. The advancements in battery technology are crucial, continually pushing boundaries and expanding the potential of what batteries can achieve.
However, these advancements come with a set of challenges.
The challenges that need to be addressed
Enhancing Energy Storage Capacity
This allows a longer driving range and makes more powerful smartphones that need less charging cycles.
Extending Battery lifespan
Efforts are directed toward minimizing the gradual degradation that batteries experience with each charge and discharge cycle. Batteries that are more stable and have a longer lifetime will reduce costs and minimize environmental waste.
Reducing charging times
The goal is to develop technologies that enable faster charging, making it as convenient as refueling a gas-powered vehicle.
Ensuring safety
Preventing battery fires and mishaps is a priority.
Minimizing scarce material dependency
Finding alternatives to scarce materials like Iridium and Platinum is essential for sustainability and cost-effectiveness.
New Battery Materials & New Battery Types
In response to these challenges, battery manufacturers are actively developing new battery materials and new types of batteries, where Atomic Layer Deposition can play a crucial role.
Layers can be achieved by using the following coatings on anode and cathode materials:
Anode materials
- Graphite; commonly used.
- Silicon; used less frequently, but upcoming!
- NMC (Oxides of Nickel, Manganese, Cobalt).
- These get used in various combinations.
“Spatial ALD is a key player in advancing battery technology, paving the way for improving cycle life and enabling the use of higher capacity anode and cathode materials”
Lithium-ion Battery
A commonly used type of rechargeable battery is the lithium-ion battery. These batteries consist of four main parts:
- The Electrodes: One Anode, One Cathode.
The anode is typically made of graphite. The cathode is typically made of iron phosphate or oxides of nickel, manganese and cobalt. - The Electrolyte
Often an extremely flammable liquid, that transports lithium ions from the cathode to the anode and vice-versa. Facilitating the batteries charge and discharge cycles. - The Seperator
A porous polymer film/membrane acts as a thin, active cell sheet that electrically isolates the anode and cathode, preventing short circuits within the battery.
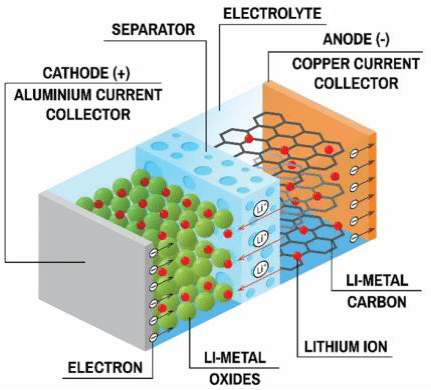

Li-ion batteries use anode and cathode materials
Next generation Li-ion batteries will use anodes made of silicon or even metallic lithium, while also new cathode materials will be used to increase the capacity of the battery. Radically new types of batteries like sodium-ion and 3D batteries are being developed to further boost performance and to reduce the use of scarce materials.
Thin film deposition
However, these new batteries require very thin protective coatings to prevent degradation of the electrodes that lead to quick deterioration of the battery’s cycle life. As these electrodes are often highly porous, ALD is one of the best methods to apply these protective films. SparkNano offers R&D tools to develop ALD coatings for new battery materials and concepts, as well as high-volume, low-cost roll-to-roll mass-production tools.
Improved battery performance
SparkNano’s Spatial ALD’s ability to deposit conformal and precise coatings is beneficial for enhancing the performance and durability of porous anodes and cathodes in batteries. Whether it involves increasing surface area for electrochemical reactions, providing protective layers, or improving ion transport within porous structures, Spatial ALD offers a versatile tool for optimizing the internal components of batteries.
